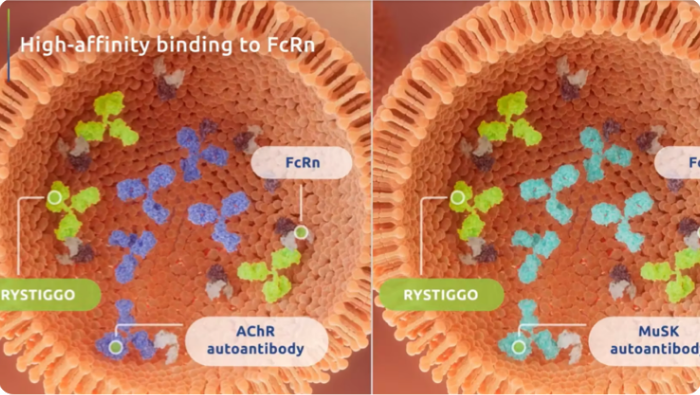
I was impressed to see data for adult patients with anti-AChR Ab+ and anti-MuSK Ab+ gMG. I encourage you to review the results from the MycarinG clinical trial.



I was impressed to see data for adult patients with anti-AChR Ab+ and anti-MuSK Ab+ gMG. I encourage you to review the results from the MycarinG clinical trial.
Dr Beth Stein is a paid consultant of UCB, Inc.
Beth Stein, MD*
Chief of Neurology
St. Joseph’s Health
Clifton, New Jersey
RYSTIGGO is the first FDA-approved targeted treatment for both anti-AChR Ab+ and anti-MuSK Ab+ adult patients with gMG.1
STUDY DESIGN
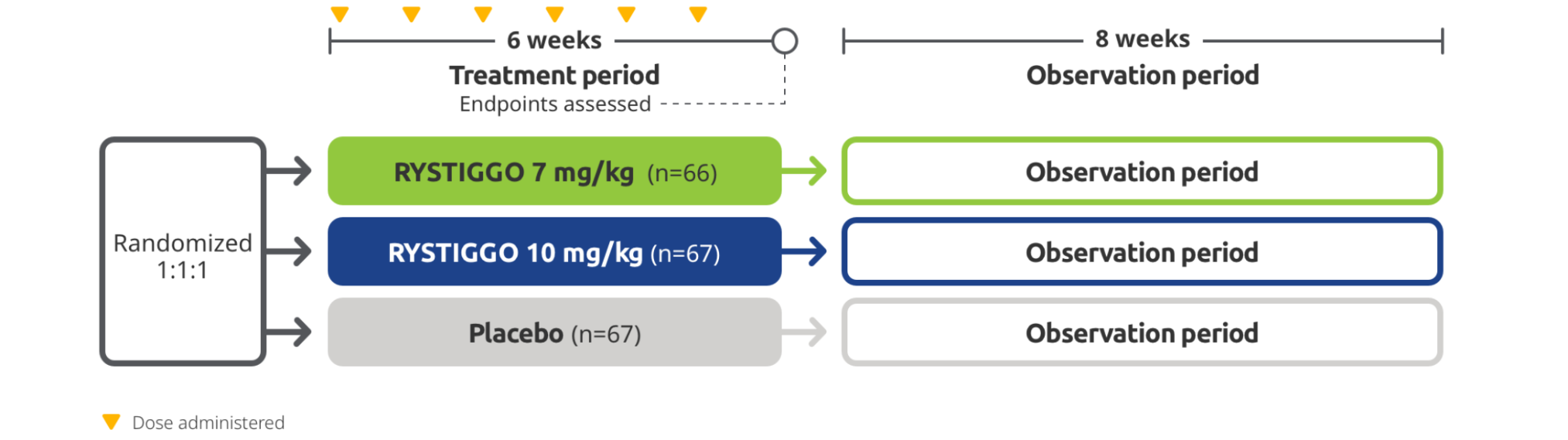
RYSTIGGO was studied in MycarinG, a large Phase 3 clinical trial of 200 adults with anti-AChR Ab+ and anti-MuSK Ab+ gMG1,2
The efficacy and safety of RYSTIGGO for the treatment of gMG were established in an 18-week, multicenter, randomized, double-blind, placebo-controlled Phase 3 study. In the study, 200 adults with gMG at least 18 years of age were randomized 1:1:1 to receive weight-tiered doses of RYSTIGGO (n=133), either 7 mg/kg of RYSTIGGO (n=66) or 10 mg/kg of RYSTIGGO (n=67), or placebo (n=67). All patients continued on current therapies.1,2
MycarinG clinical trial1,2
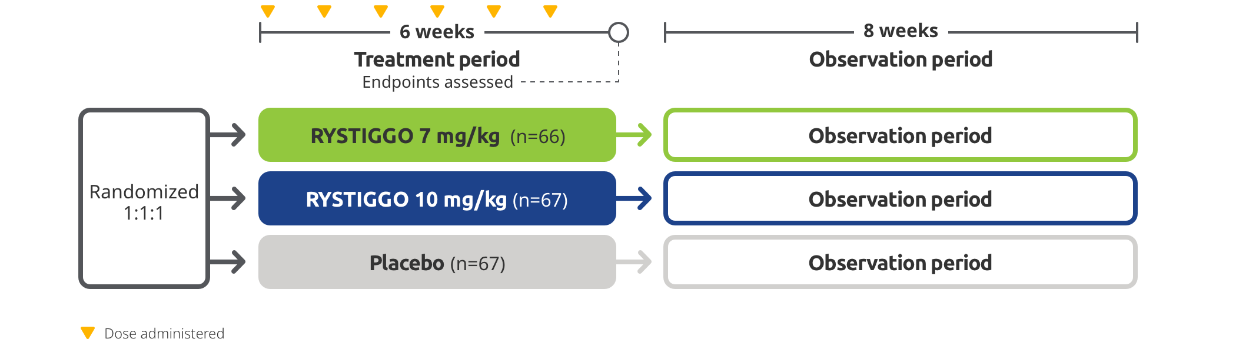
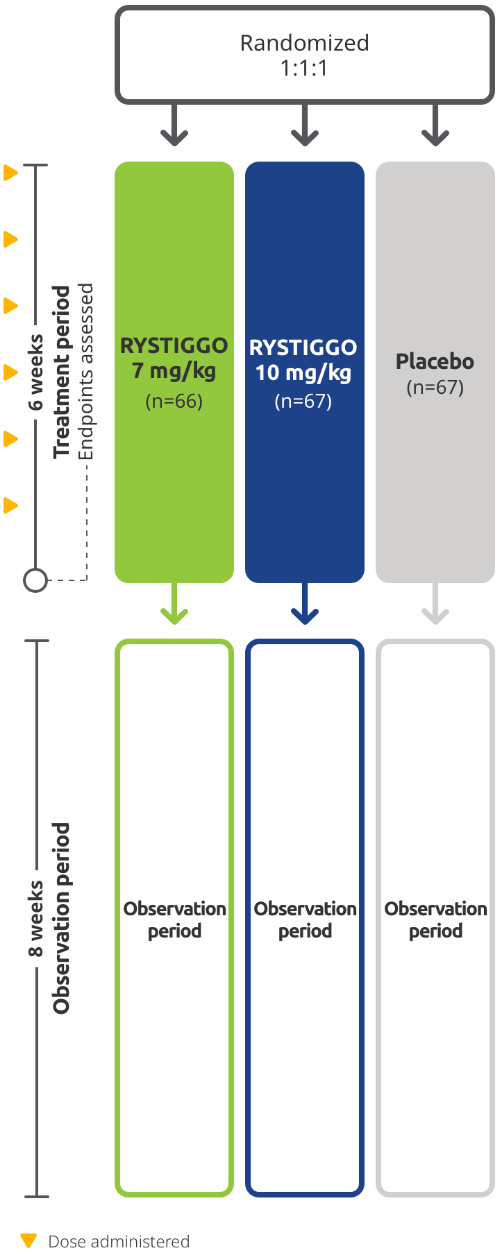
Baseline Characteristics2,3:
|
RYSTIGGO 7 mg/kg (n=66) |
RYSTIGGO 10 mg/kg (n=67) |
Placebo (n=67) |
|
|---|---|---|---|
| Mean age in years | 53 | 52 | 50 |
| Median duration of disease in years | 5.3 | 5.7 | 6.8 |
| Gender distribution, % female | 59% | 52% | 70% |
| Race, % White | 62% | 73% | 69% |
| Race, % Asian | 14% | 10% | 7% |
| Race, % Black | 0% | 6% | 1% |
| Race, % American Indian or Alaska Native | 0% | 0% | 0% |
| Ethnicity, % Hispanic or Latino | 8% | 5% | 8% |
| Mean MG-ADL total score | 8.4 | 8.1 | 8.4 |
| Mean QMG total score | 15.4 | 15.6 | 15.8 |
| Anti-AChR Ab+, n (%) | 60 (91%)* | 60 (90%) | 59 (88%)* |
| Anti-MuSK Ab+, n (%) | 5 (8%) | 8 (12%)† | 8 (12%)† |
| Treatment with acetylcholinesterase inhibitors, n (%) | 55 (83%) | 57 (85%) | 60 (90%) |
| Treatment with steroids, n (%) | 43 (65%) | 48 (72%) | 38 (57%) |
| Treatment with non-steroidal immunosuppressive therapies, n (%) | 32 (48%) | 38 (57%) | 33 (49%) |
*Includes 1 patient who had unknown AChR and MuSK autoantibody status.2
†Includes 1 patient who had positive AChR and MuSK autoantibody status.2
The clinical study included adults who were anti-AChR Ab+ and anti-MuSK Ab+, and who had MGFA class II-IVa disease, an MG-ADL score of ≥3 (with ≥3 points from non-ocular symptoms), serum immunoglobulin G levels of ≥5.5 g/L, and who were on a stable dose of MG therapy prior to screening.1
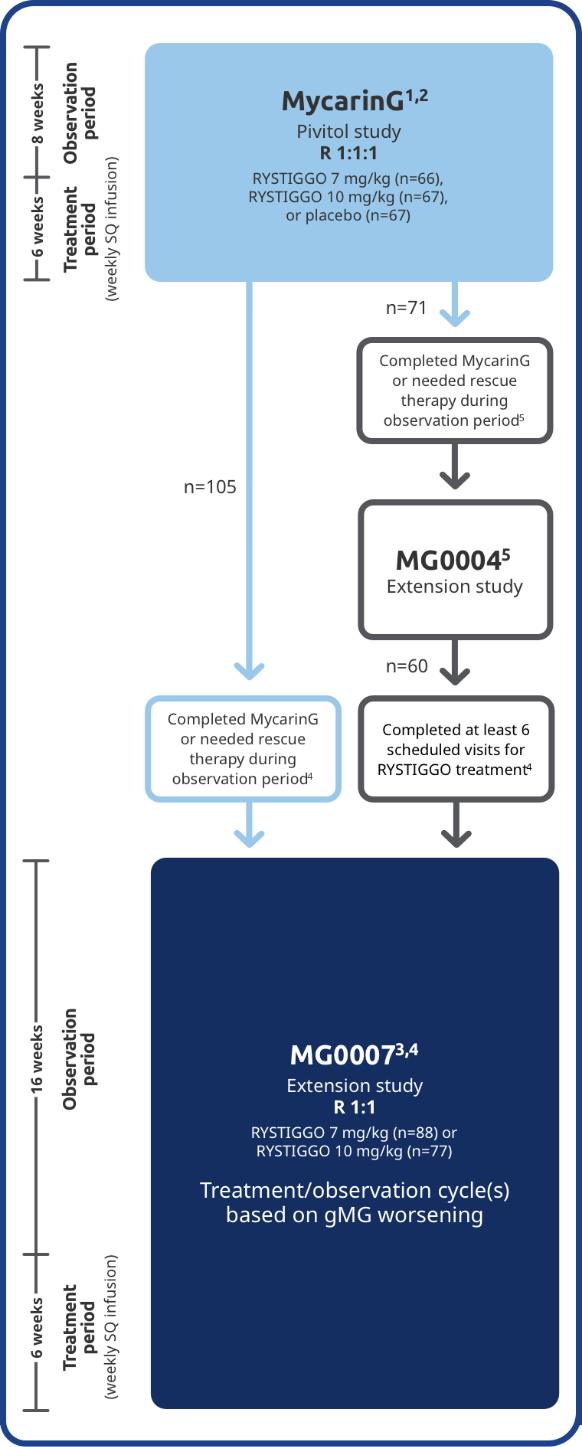
EXTENSION STUDIES
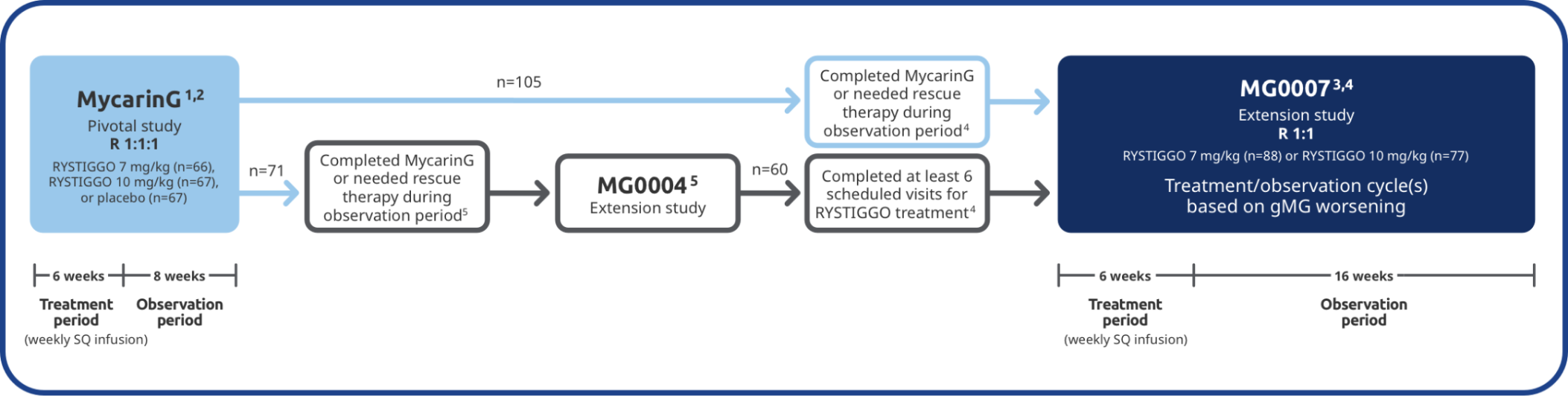
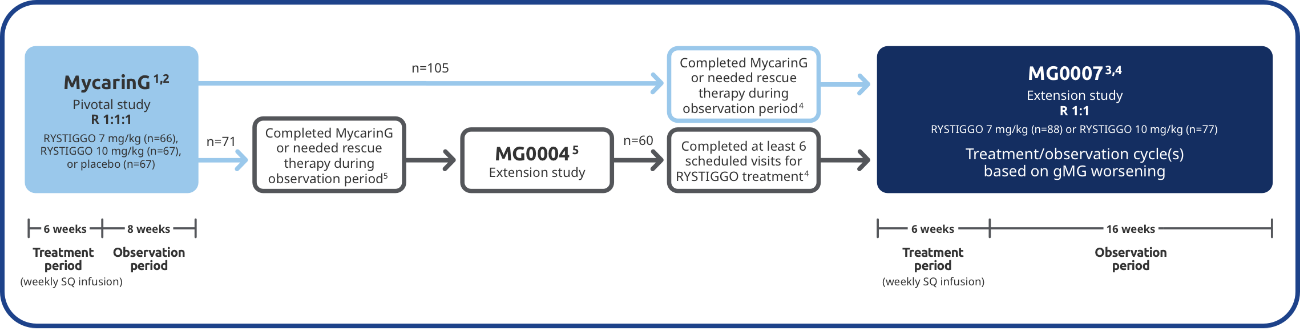
The long-term safety and efficacy of RYSTIGGO were evaluated in adults in two extension studies4
Adults from MycarinG could enroll in two extension studies, MG0004 and MG0007, that further evaluated the safety and efficacy of RYSTIGGO over time.4
MG0004 (NCT04124965)
MG0004 was an up to 60-week, multicenter, randomized, Phase 3 extension study. 71 adult patients from MycarinG who met eligibility criteria were randomized to receive either RYSTIGGO 7 mg/kg or 10 mg/kg. Patients received RYSTIGGO once weekly for a period of up to 52 weeks, followed by 8 weeks of observation. MG0004 enrollment was closed upon the initiation of study MG0007.4
MG0007 (NCT04650854)
MG0007 was a multicenter Phase 3 extension study that included 165 adults from MycarinG and MG0004. It assessed the long-term safety and efficacy of RYSTIGGO given in repeated 6-week cycles, based on the manifestation of gMG symptoms.3,4
EFFICACY
Primary Endpoint: Change from baseline to Week 6 (Day 43) in MG-ADL total score in adults who are anti-AChR Ab+ or anti-MuSK Ab+1-3*
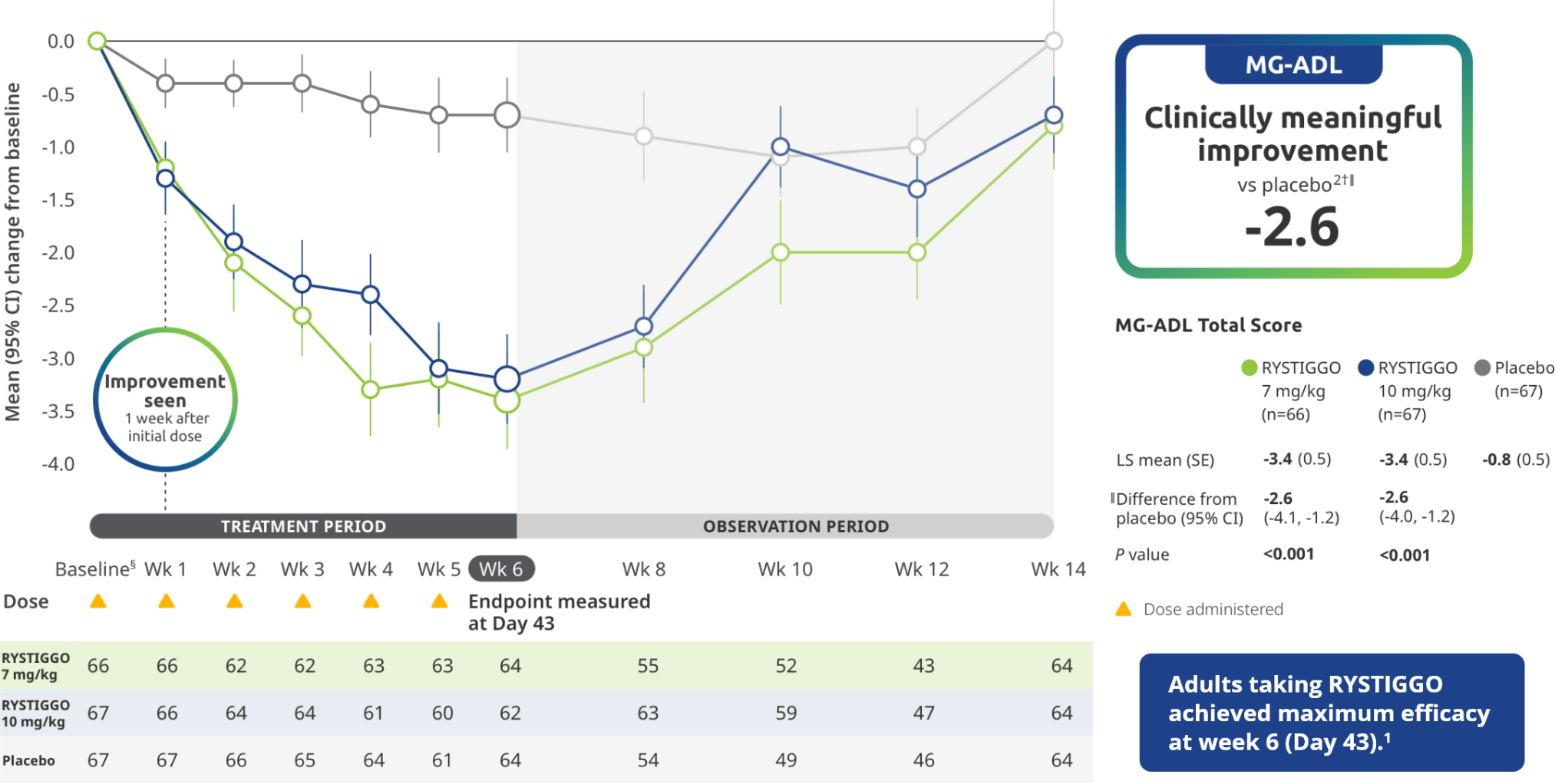
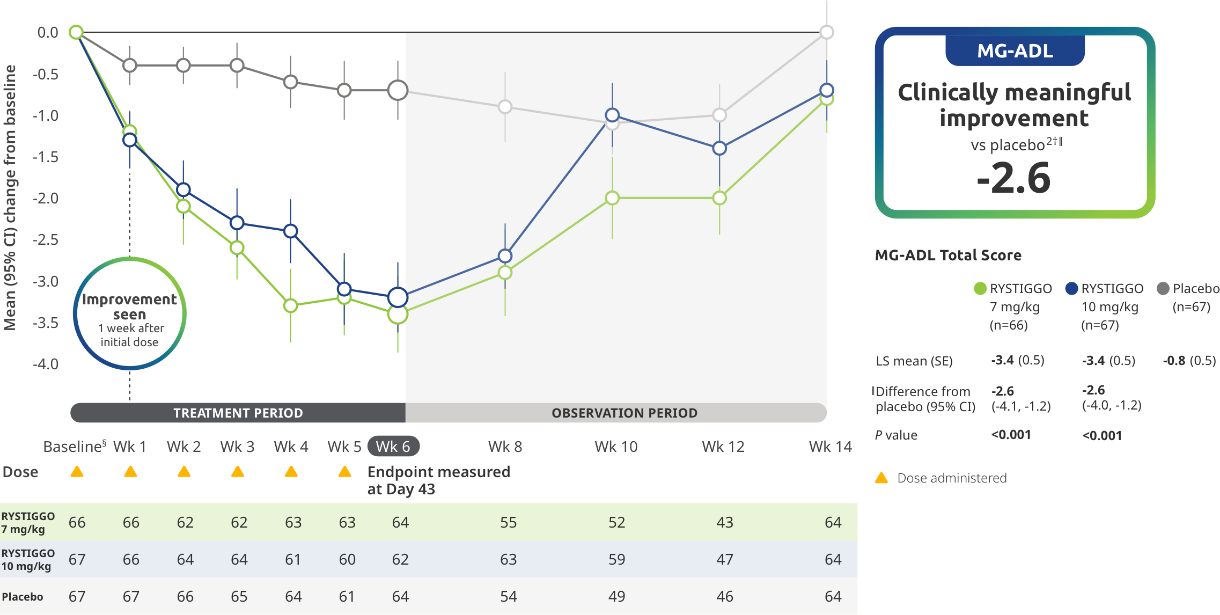
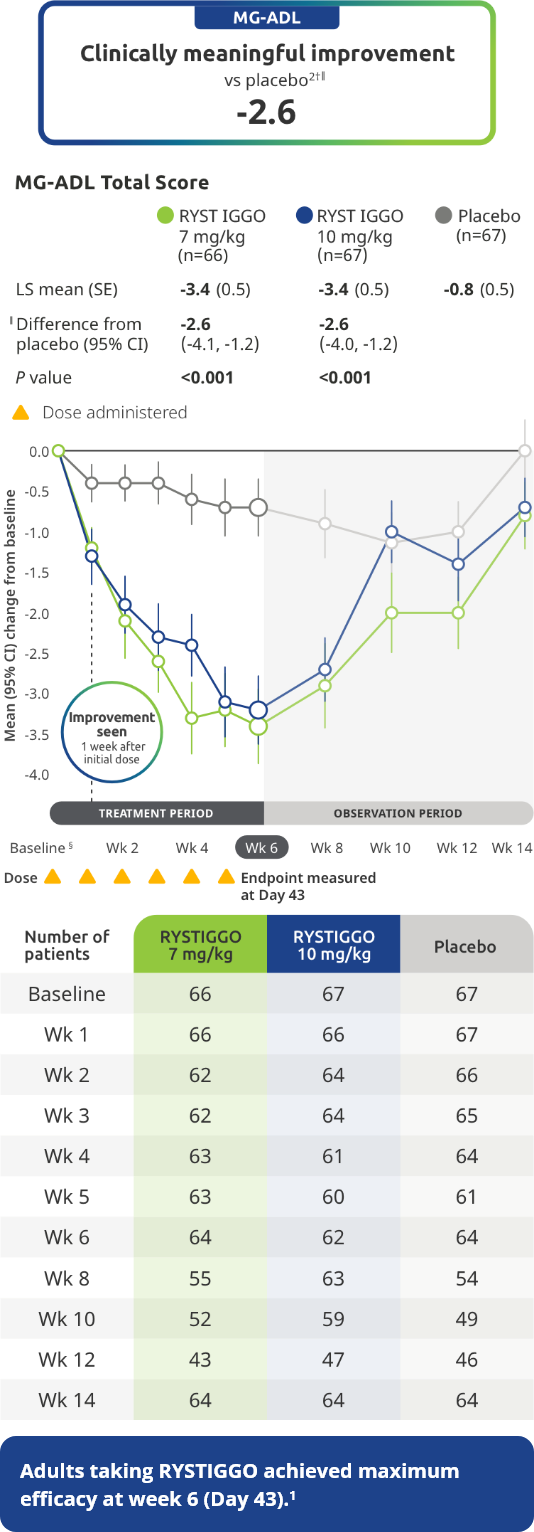
The efficacy of RYSTIGGO in adults who are anti-AChR Ab+ or anti-MuSK Ab+ was established in a multicenter, randomized, double-blind, placebo-controlled study. The study included a 4-week screening period and a 6-week treatment period followed by 8 weeks of observation.1
During the treatment period, RYSTIGGO or placebo was administered subcutaneously once a week for 6 weeks.1
Treatment initiation on Day 1.2
Clinically meaningful was established as a ≥2.0-point improvement in MG-ADL total score.2
The most common adverse reactions (≥10%) in adults treated with RYSTIGGO were headache, infections, diarrhea, pyrexia, hypersensitivity reactions, and nausea.1
Proportion of MG-ADL responders from baseline at Week 62
Secondary endpoint: MG-ADL responder rate:
Adults with a ≥2.0-point improvement in MG-ADL total score from baseline at Week 6 (Day 43)2
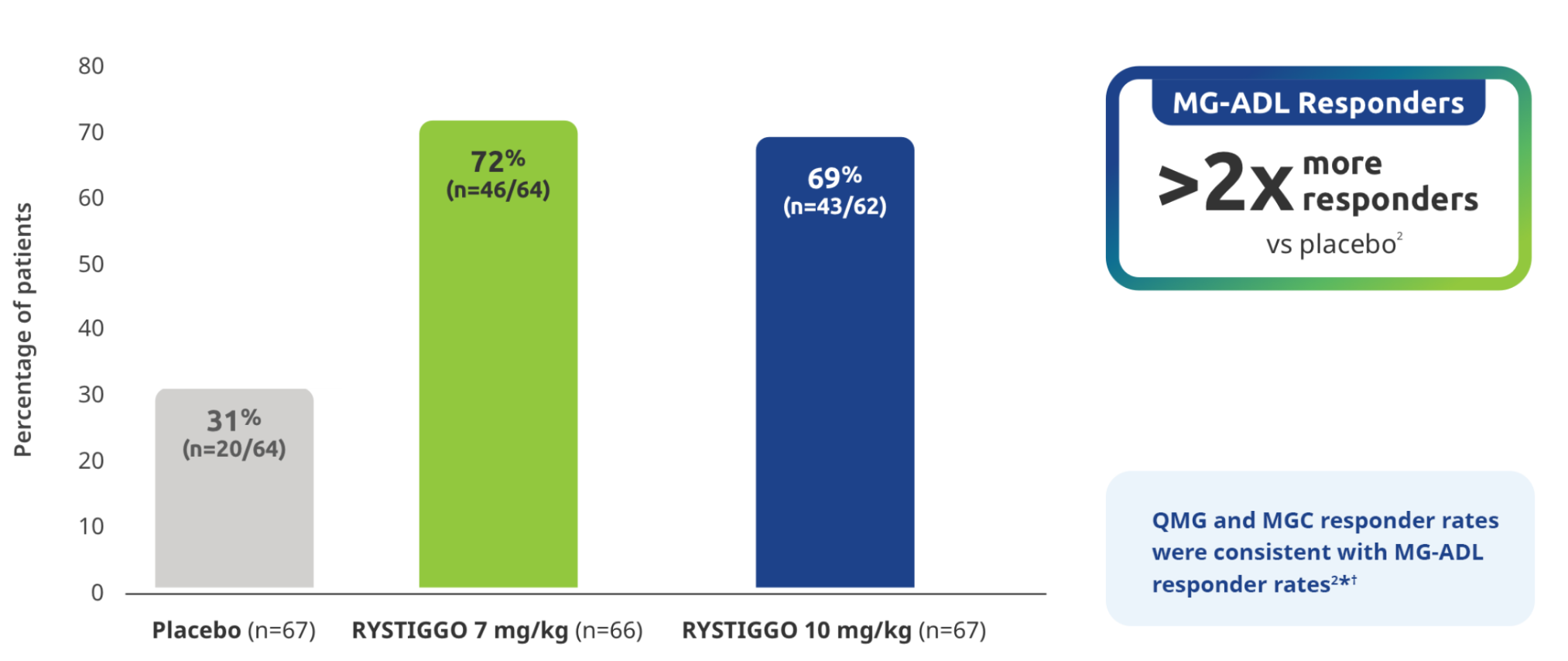
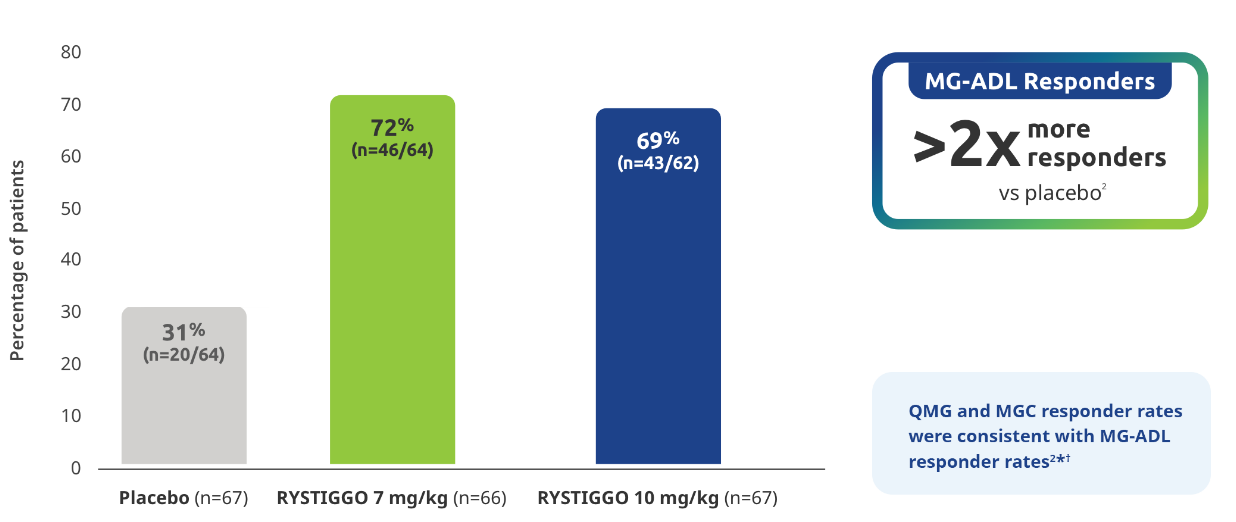
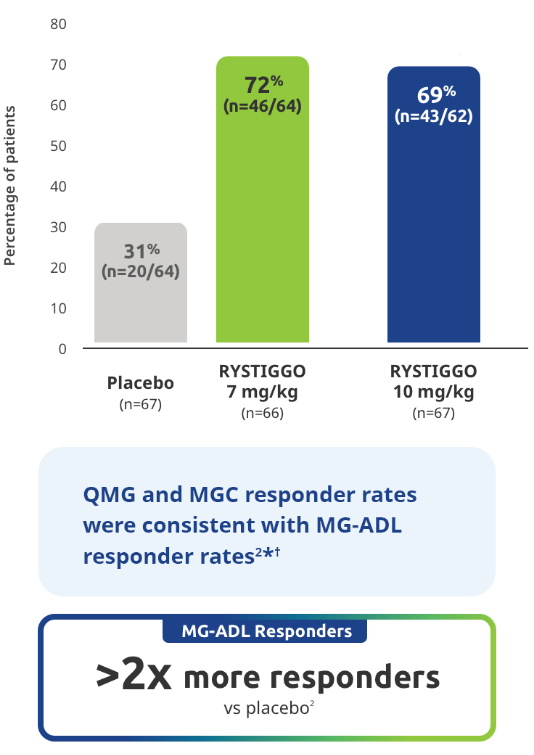
Study limitations: MG-ADL responder rate was a prespecified secondary endpoint not controlled for multiplicity; therefore, data should be interpreted with caution and conclusions cannot be drawn.
*QMG and MGC responders were established as adults with a ≥3.0-point improvement in QMG and MGC score from baseline at Week 6 (Day 43).2
QMG responder rates from baseline at Week 6 (Day 43) were 54.7% (n=35/64) in the RYSTIGGO 7 mg/kg group and 72.6% (n=45/62) in the RYSTIGGO 10 mg/kg group compared to 39.1% (n=25/64) in the placebo group. MGC responder rates from baseline at Week 6 (Day 43) were 60.9% (n=39/64) in the RYSTIGGO 7 mg/kg group and 74.2% (n=46/62) in the RYSTIGGO 10 mg/kg group compared to 40.6% (n=26/64) in the placebo group.2
The first treatment indicated for adults with anti-MuSK Ab+ gMG1
Subgroup analysis of the primary efficacy endpoint:
Mean change from baseline to Week 6 (Day 43) in MG-ADL total score in adults with anti–MuSK Ab+ gMG5
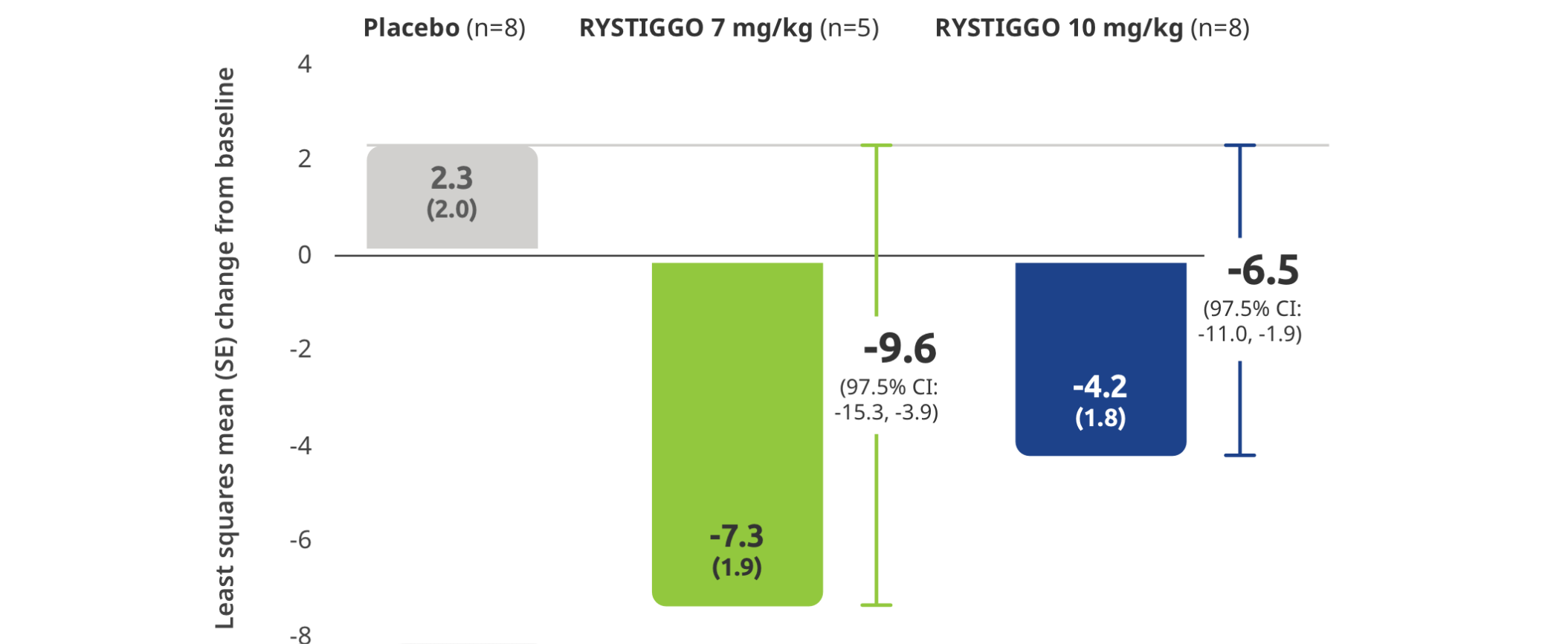
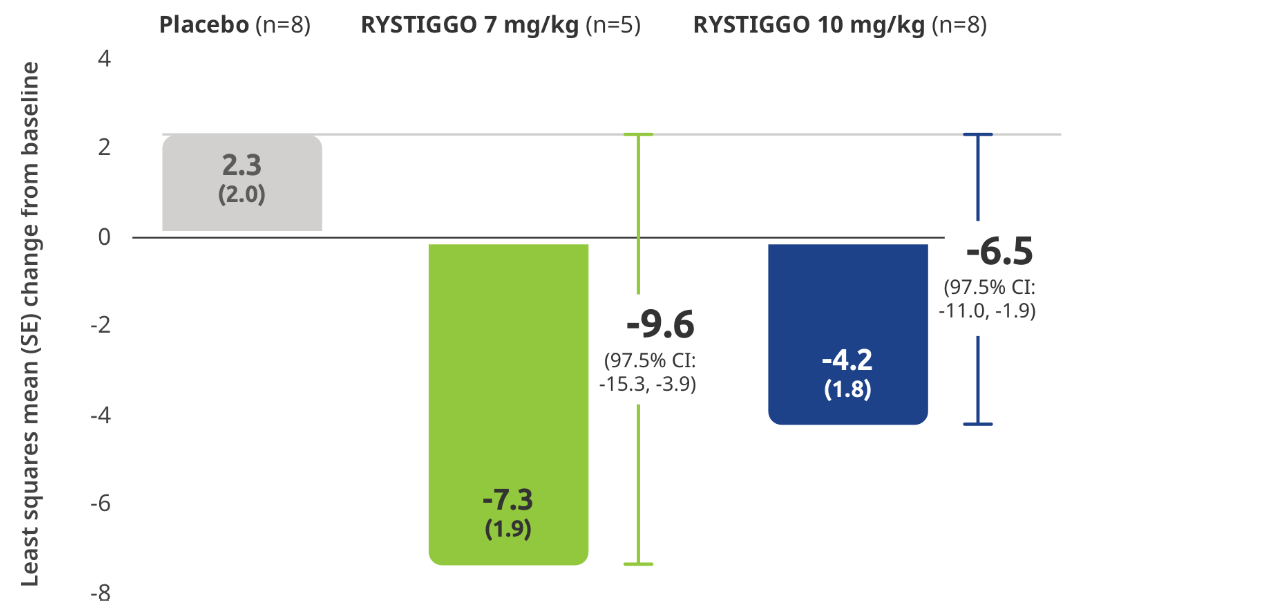
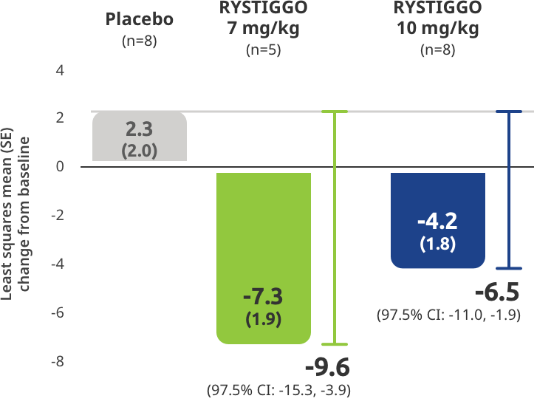
All subgroup analyses were descriptive.5
All adults with anti-MuSK Ab+ gMG who received RYSTIGGO and had data available at Week 6 were MG-ADL responders2
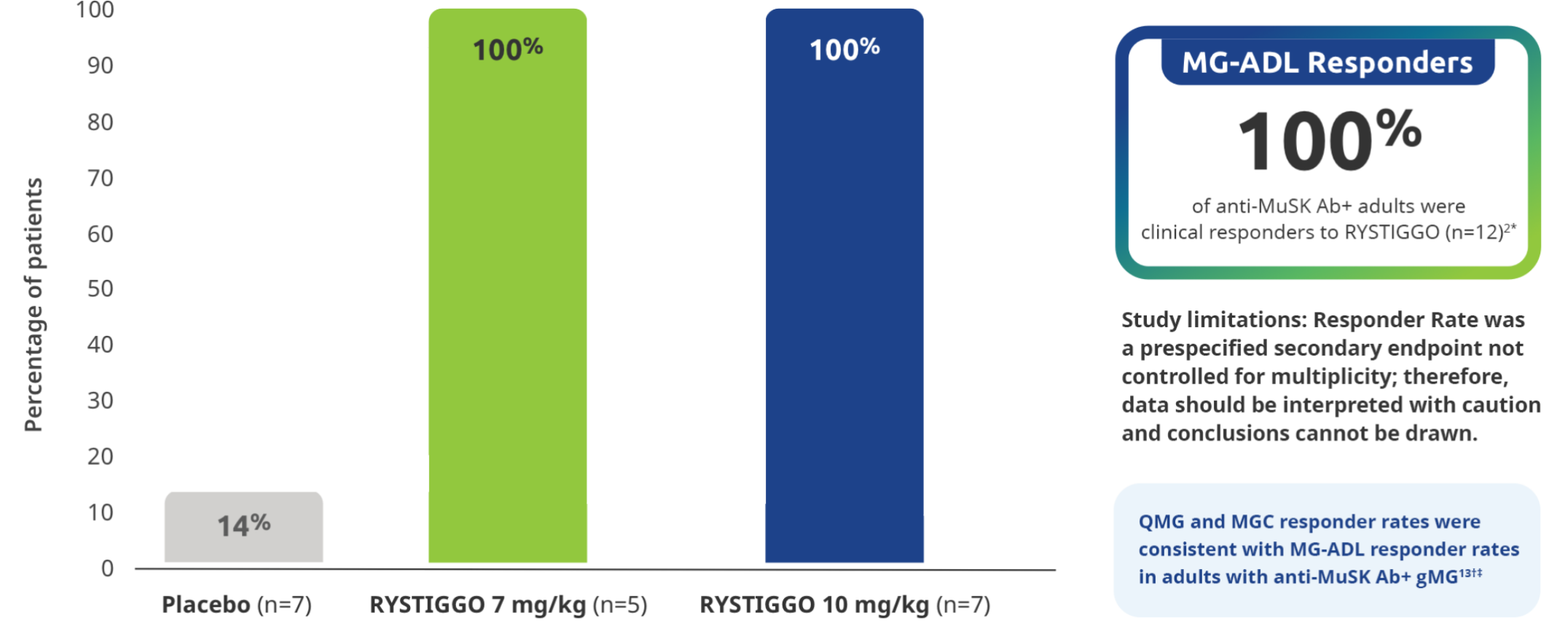
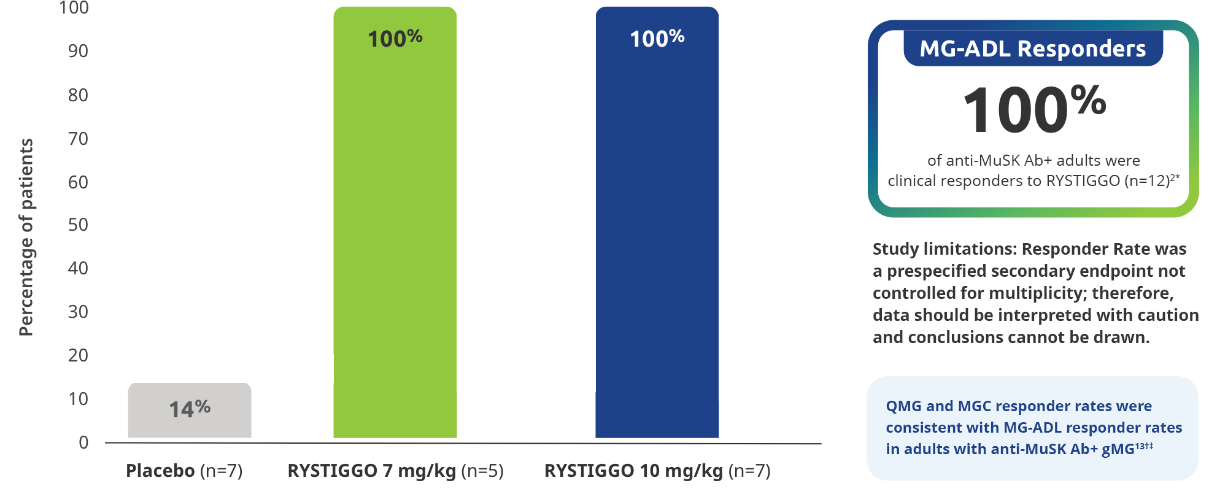
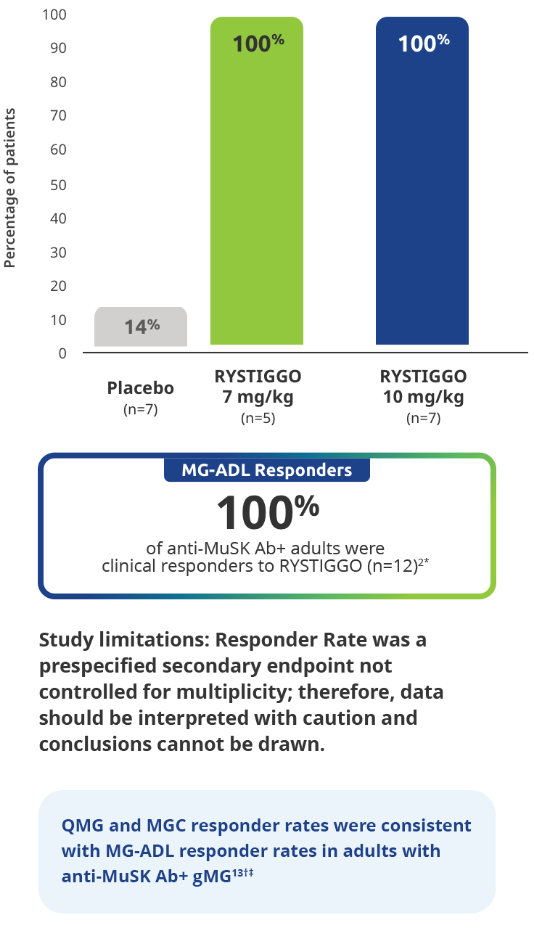
All subgroup analyses were descriptive.5
Clinical responders were established as adults with a ≥2.0-point improvement in the total MG-ADL score compared to baseline at Week 6 (Day 43). Clinical responder data were not collected for 1 patient in the RYSTIGGO 10 mg/kg group who discontinued treatment.2,3
QMG and MGC responders were established as adults with a ≥3.0-point improvement in QMG and MGC score from baseline at Week 6 (Day 43).5
QMG responder rates from baseline at Week 6 (Day 43) were 100% (n=5/5) in the RYSTIGGO 7 mg/kg group and 85.7% (n=6/7) in the RYSTIGGO 10 mg/kg group compared to 28.6% (n=2/7) in the placebo group. MGC responder rates from baseline at Week 6 (Day 43) were 100% (n=5/5) in the RYSTIGGO 7 mg/kg group and 100% (n=7/7) in the RYSTIGGO 10 mg/kg group compared to 0.0% (n=0/7) in the placebo group.5
OTHER MG-ADL EFFICACY ENDPOINT
Minimal Symptom Expression (MSE) rates observed during the pivotal study2
Other efficacy endpoint: Proportion of adults achieving MSE during treatment and observation in the pivotal study2
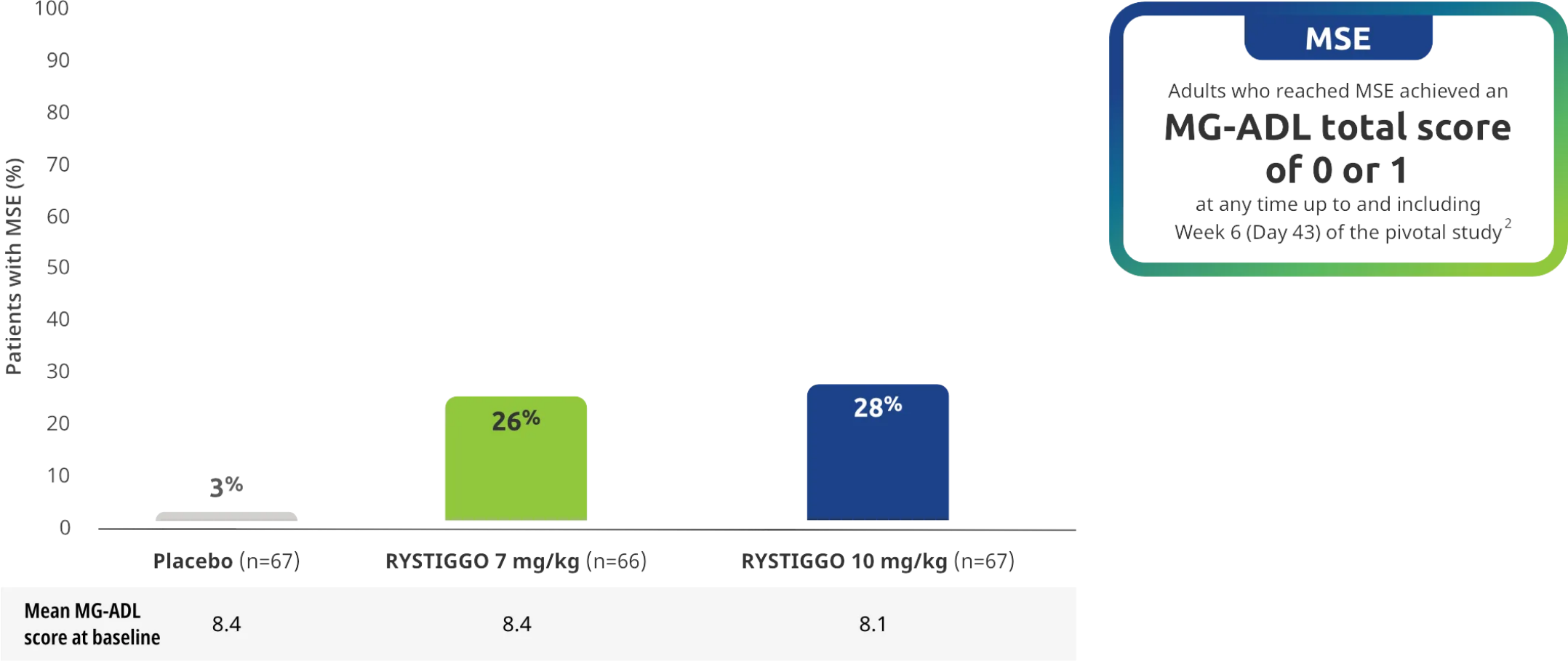
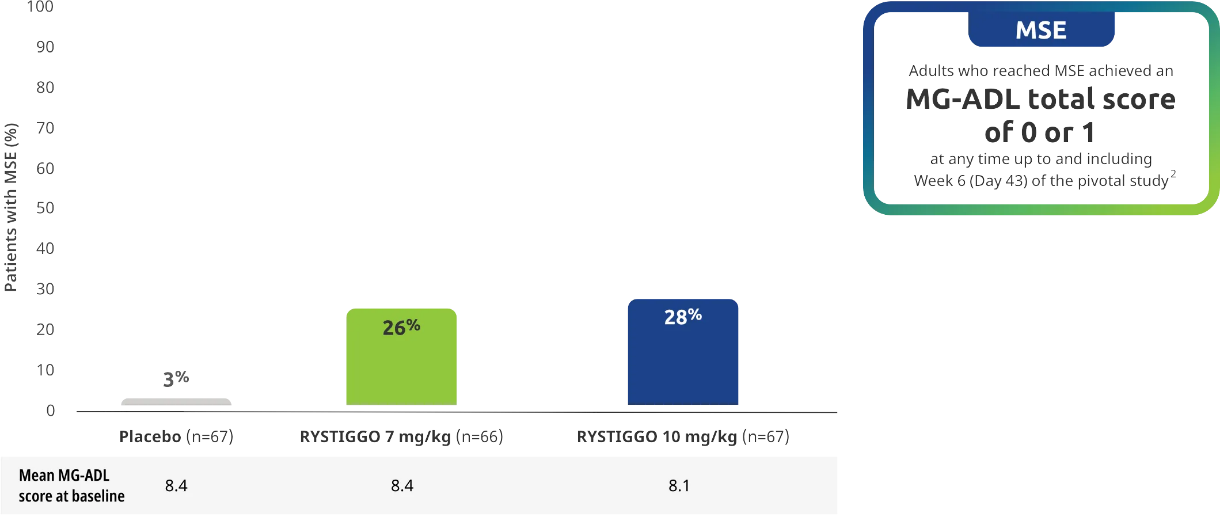
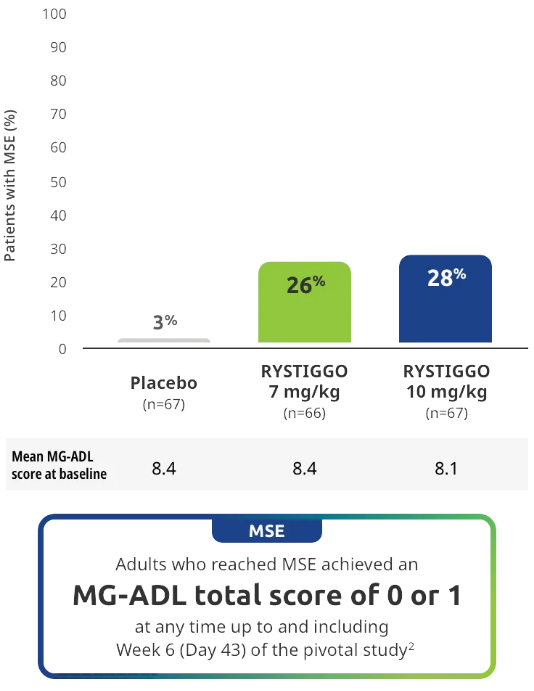
All subgroup analyses were descriptive.
MSE was an exploratory endpoint and not controlled for multiplicity. Therefore, data should be interpreted with caution and conclusions cannot be drawn.
MG-ADL SCORES IN EXTENSION STUDIES
Consistent improvements in MG-ADL scores across the pivotal study and subsequent cycles of RYSTIGGO in the extension studies1,6
MG-ADL total scores from baseline to Week 6 (Day 43) across each subsequent 6-week treatment cycle3,6*
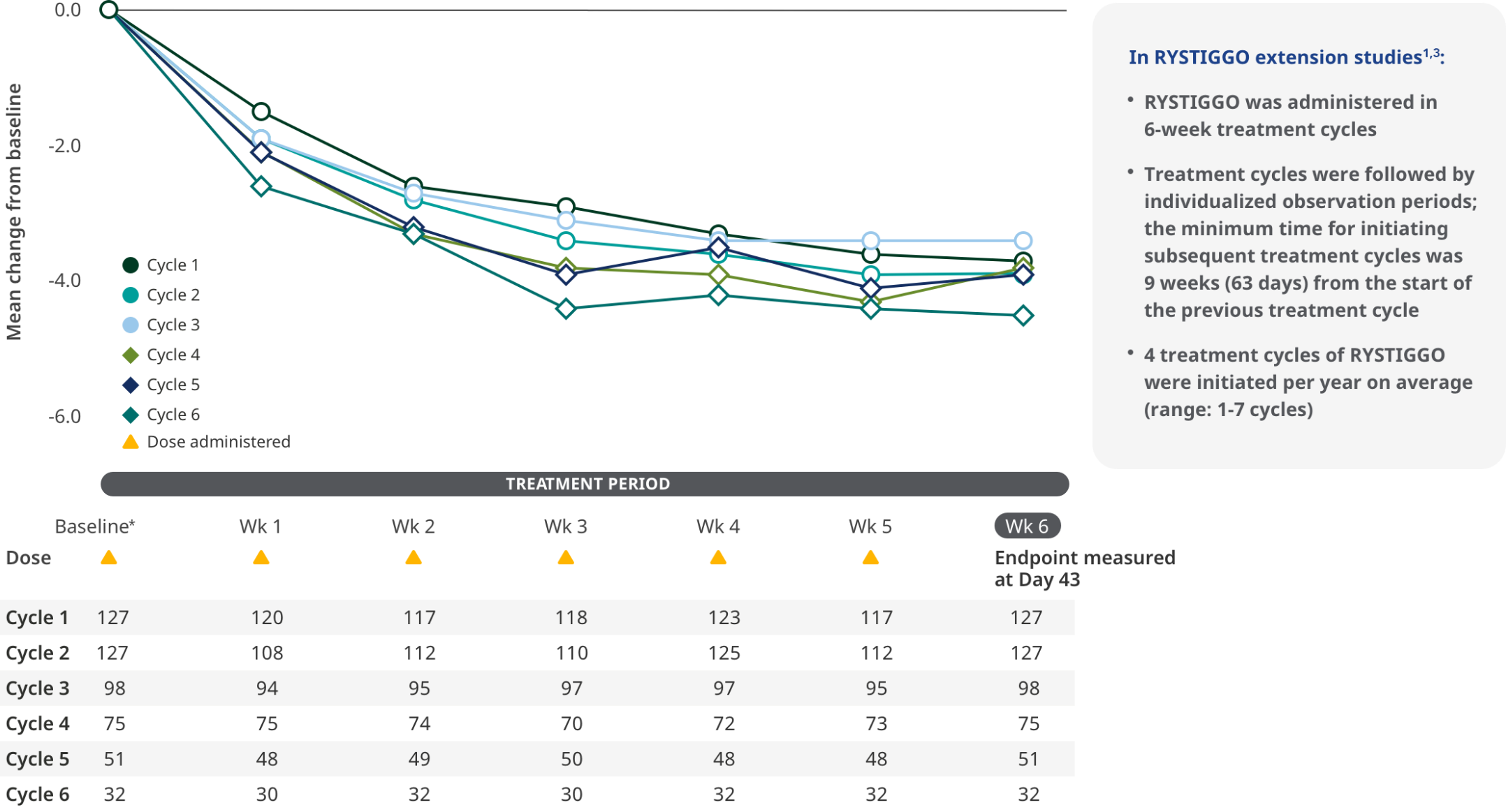
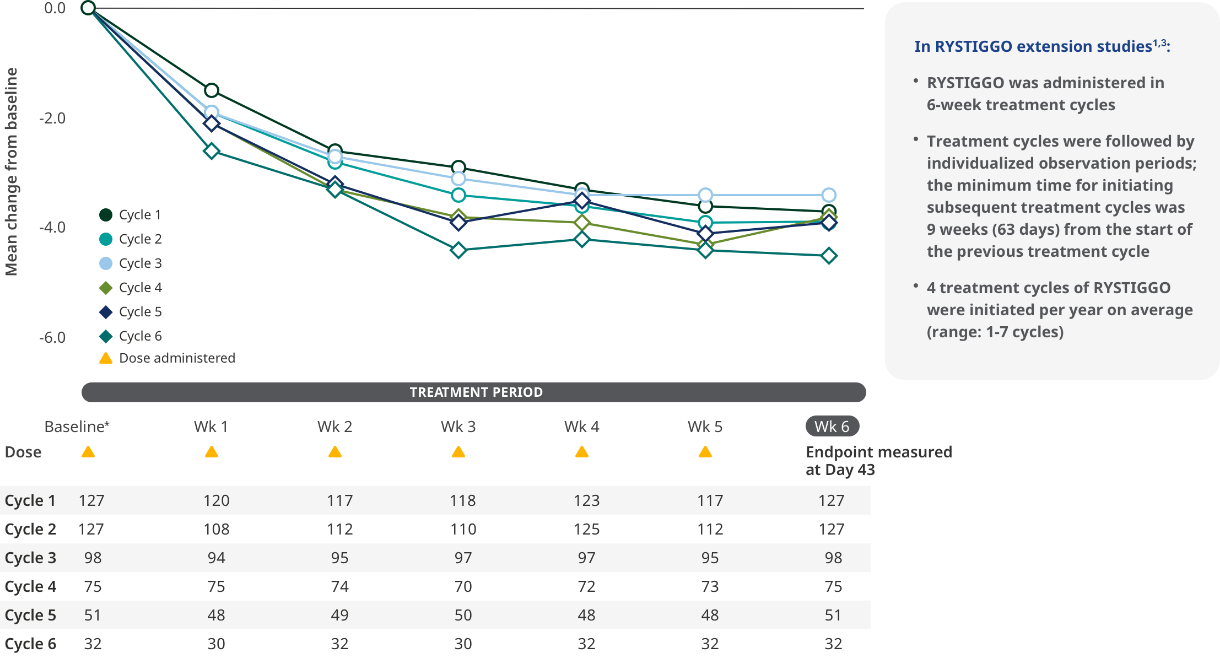
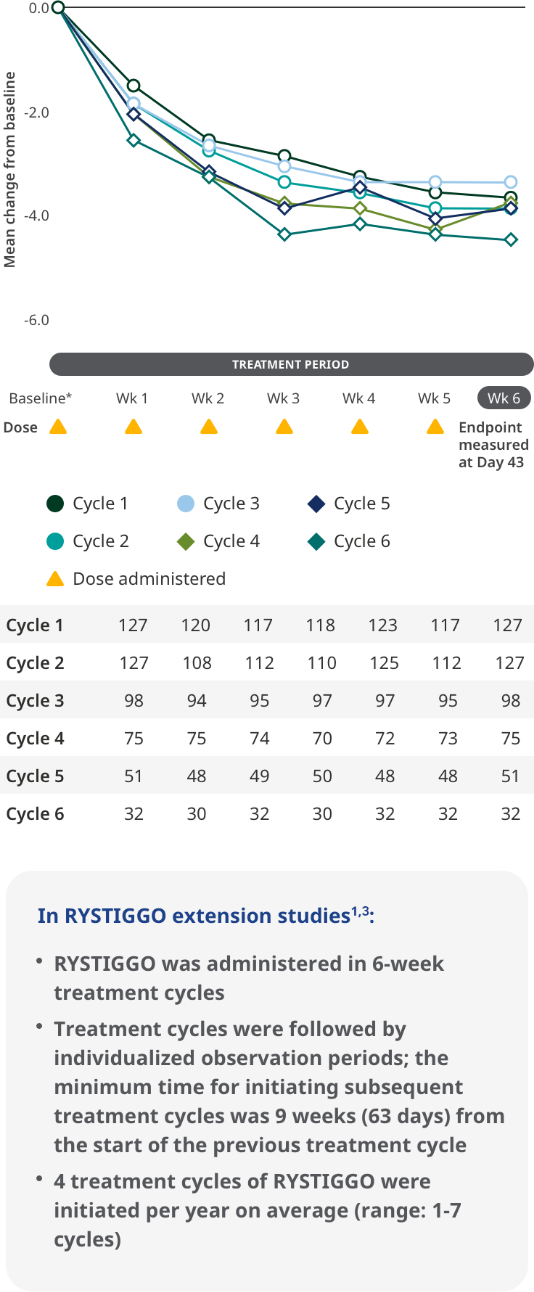
The most common adverse reactions observed with repeated cyclic treatment were consistent with adverse reactions observed in the pivotal study1,3
The RYSTIGGO extension studies were open label and not placebo controlled; therefore, the efficacy and clinical significance of the results should be interpreted with caution.
Data were pooled across MycarinG, the first 6 weeks of MG0004, and MG0007 and combined for the 7 mg/kg and 10 mg/kg RYSTIGGO treatment groups.6
Clinically meaningful was established as a ≥2.0-point improvement in MG-ADL total score.6
Treatment initiation on Day 1.4
MSE IN EXTENSION STUDIES
MSE rates through subsequent cycles of RYSTIGGO7
Exploratory endpoint: Proportion of adults achieving MSE with each subsequent cycle of RYSTIGGO2,7*†
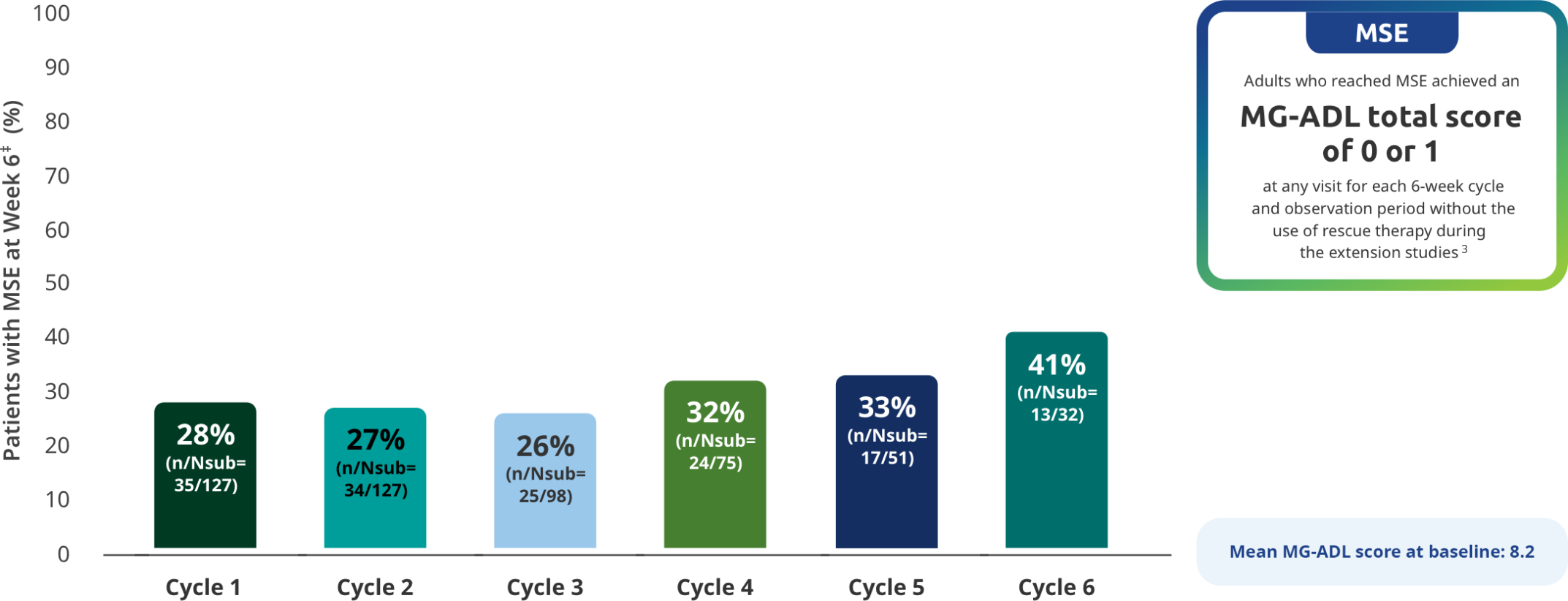
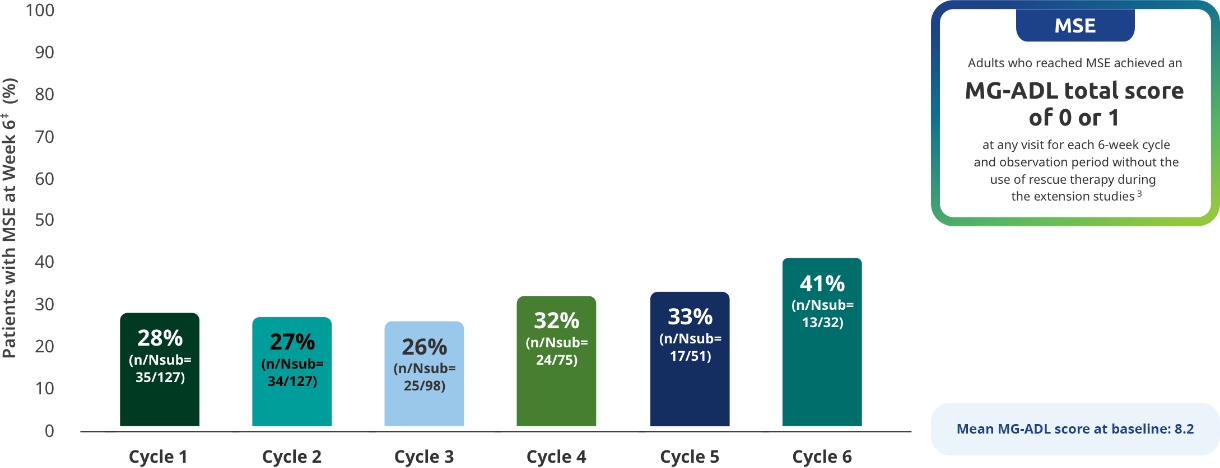
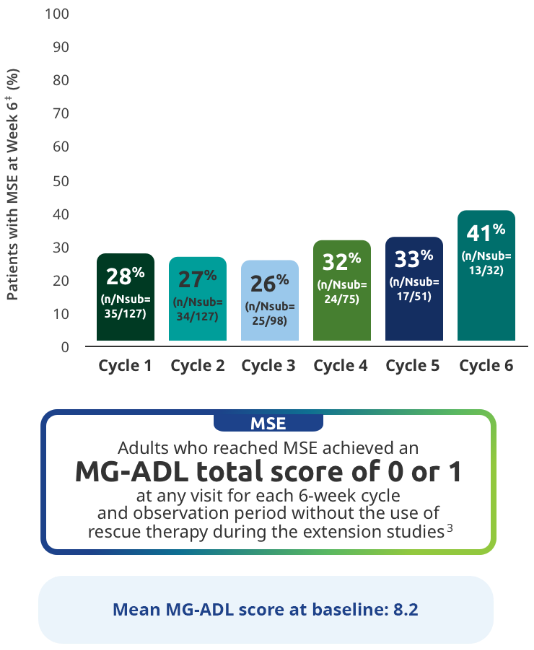
The RYSTIGGO extension studies were open label and not placebo controlled; therefore, the efficacy and clinical significance of the results should be interpreted with caution.
MSE was an exploratory endpoint and not controlled for multiplicity. Therefore, data should be interpreted with caution and conclusions cannot be drawn.
Data should be interpreted with caution given the decreasing number of patients receiving subsequent treatment cycles.
*Data were pooled across MycarinG, the first 6 weeks of MG0004, and MG0007 and combined for the 7 mg/kg and 10 mg/kg RYSTIGGO treatment groups.7
N represents the number of adults with MSE assessment in the efficacy pool at each treatment cycle.7
Day 43.
TREATMENT BREAKS
The majority of adults had treatment breaks between 4 and <8 weeks in the extension studies3
Time to subsequent symptom-driven treatment cycle from last subcutaneous infusion2,3*
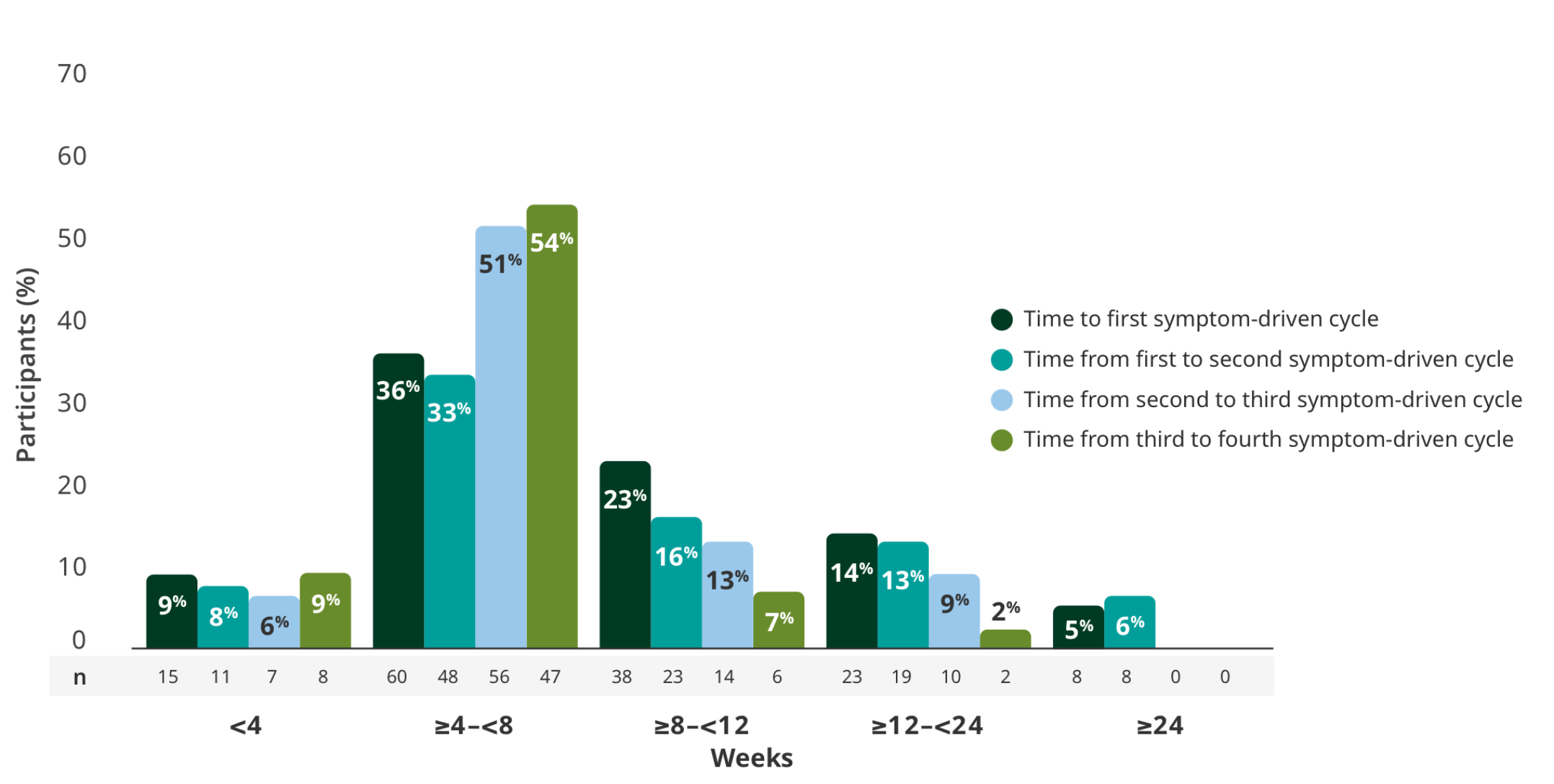
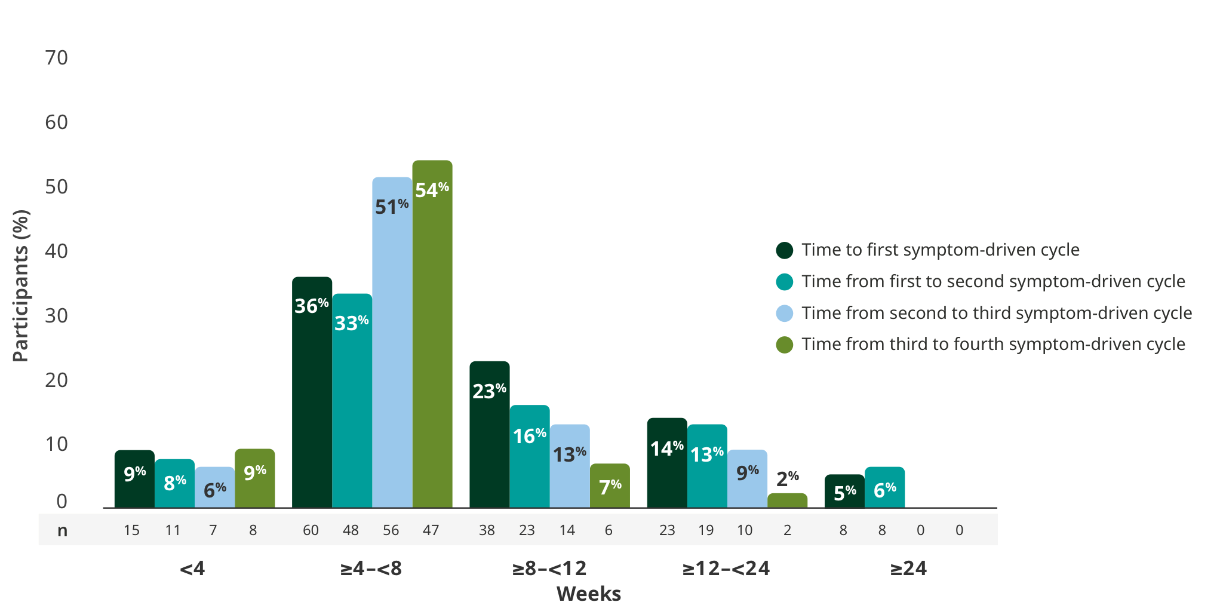
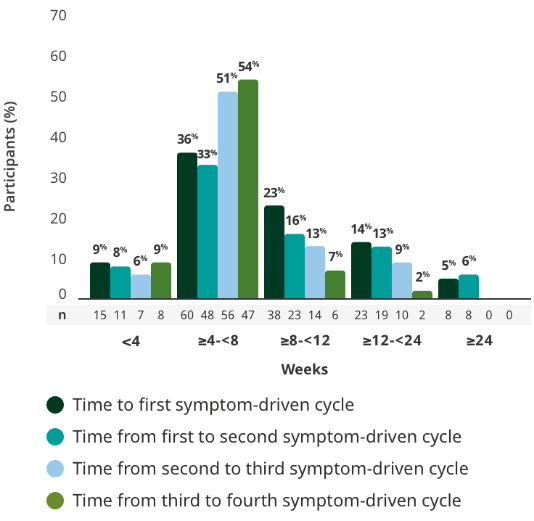
Interim analysis as of July 8, 2022.2
- The median time between treatment cycles was 8 weeks (56 days) for adults treated with RYSTIGGO who initiated 4 cycles1†
- The safety of initiating subsequent cycles sooner than 9 weeks (63 days) from the start of the previous treatment cycle has not been established1
- The most common adverse reactions being observed with repeated cyclic treatment were consistent with adverse reactions observed in the pivotal study1,3
The RYSTIGGO extension studies were open label and not placebo controlled; therefore, the efficacy and clinical significance of the results should be interpreted with caution.
Data were pooled across MycarinG, the first 6 weeks of MG0004, and MG0007 and combined for the 7 mg/kg and 10 mg/kg RYSTIGGO treatment groups.2
This is equivalent to 14 weeks (98 days) from the start of the previous treatment cycle.1
SAFETY
MycarinG CLINICAL TRIAL
RYSTIGGO safety was demonstrated in MycarinG, a Phase 3 clinical study1,2
| Adverse Reaction | RYSTIGGO (n=133) |
Placebo (n=67) |
|---|---|---|
| Headache | 44% | 19% |
| Any infection | 23% | 19% |
| Upper respiratory tract infection | 8% | 6% |
| Diarrhea | 20% | 13% |
| Pyrexia | 17% | 2% |
| Hypersensitivity reactions | 11% | 5% |
| Nausea | 10% | 8% |
| Administration site reactions | 8% | 3% |
| Abdominal pain | 8% | 6% |
| Arthralgia | 7% | 3% |
The most common adverse reactions (≥10%) in adults treated with RYSTIGGO were headache, infections, diarrhea, pyrexia, hypersensitivity reactions, and nausea.1
RYSTIGGO Warnings and Precautions
RYSTIGGO may increase the risk of infection1*
In MycarinG and the extension studies, out of 196 patients treated with RYSTIGGO,
94 (48%) patients reported infection
Common infections (at least 5% frequency) were upper respiratory tract infections (17%), COVID-19 (14%), urinary tract infections (9%), and herpes simplex (6%)
Serious infections were reported in 4% of patients treated with RYSTIGGO
Three fatal cases of pneumonia were identified caused by COVID-19 infection in two patients and an unknown pathogen in one patient
Six cases of infections led to discontinuation of RYSTIGGO
Delay administration of RYSTIGGO to patients with an active infection1
Monitor for signs and symptoms of infection in patients treated with RYSTIGGO1
If serious infection occurs, administer appropriate treatment and consider withholding RYSTIGGO until the infection has resolved1
Immunization with live-attenuated or live vaccines is not recommended during treatment with RYSTIGGO.1
Aseptic meningitis has been reported in patients treated with RYSTIGGO1
Serious adverse reactions of aseptic meningitis (also called drug-induced aseptic meningitis) have been reported in patients treated with RYSTIGGO. If symptoms consistent with aseptic meningitis develop, diagnostic workup and treatment should be initiated according to the standard of care.
Hypersensitivity reactions were observed in patients treated with RYSTIGGO1
In clinical trials, hypersensitivity reactions, including angioedema and rash, occurred within 1 day to 2 weeks of administration
One patient discontinued RYSTIGGO due to a hypersensitivity reaction
Local reactions at the administration site occurred within 1 to 3 days after the most recent RYSTIGGO infusion
Monitor patients during administration and for 15 minutes after completion for clinical signs and
symptoms of hypersensitivity reactions1
If a hypersensitivity reaction occurs during administration, the healthcare professional should
institute appropriate measures if needed or the patient should seek medical attention1
EXTENSION STUDIES
RYSTIGGO safety was further evaluated in the extension studies, with primary endpoints assessing the proportion of patients experiencing TEAEs8,9
TEAEs reported in >10% of adults in at least one of the RYSTIGGO treatment groups3
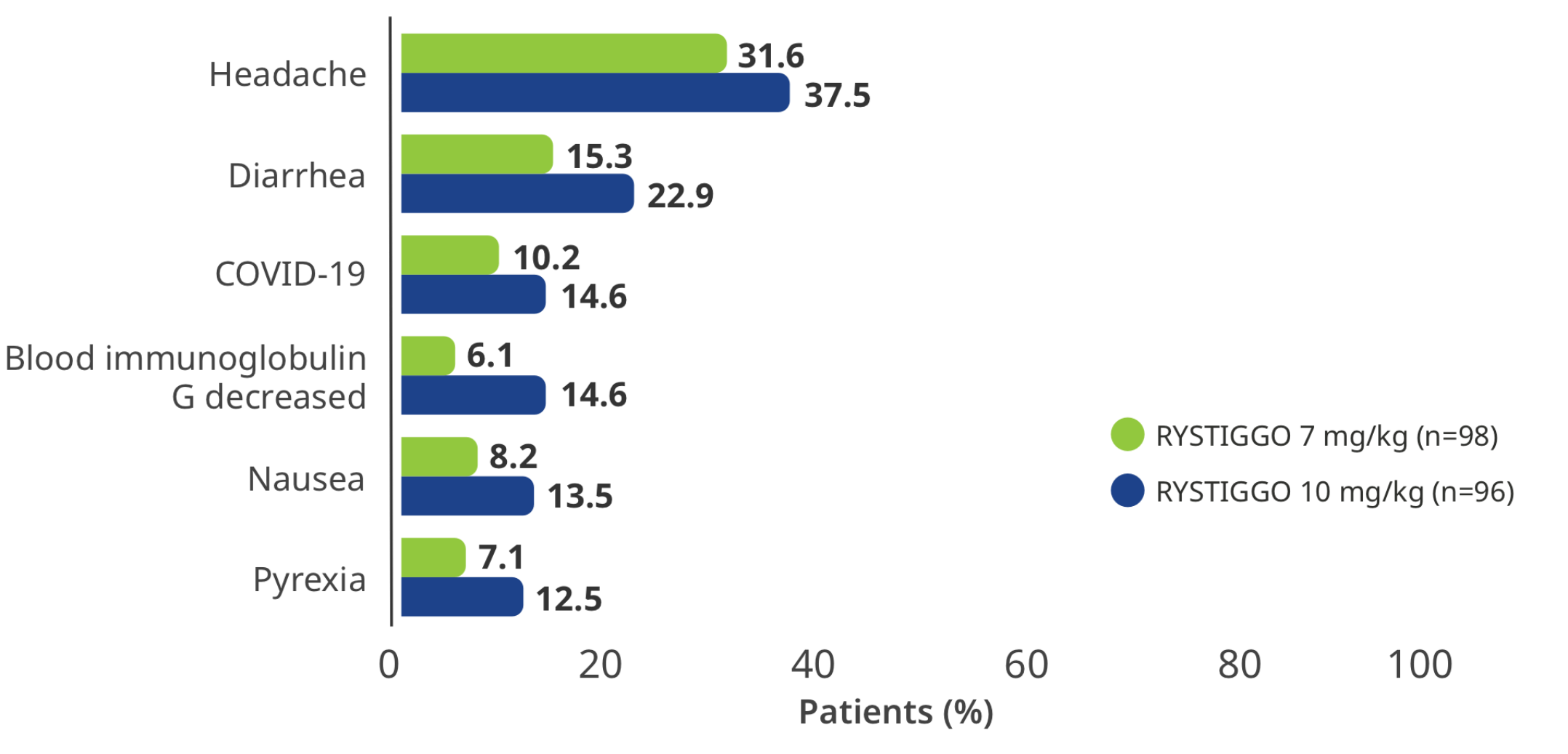
Interim analysis as of July 8, 2022.3
Ab+=antibody positive; AChE=acetylcholinesterase; AChR=acetylcholine receptor; COVID-19=coronavirus disease 2019; gMG=generalized myasthenia gravis; IgG=immunoglobulin G; MG-ADL=Myasthenia Gravis Activities of Daily Living; MGC=Myasthenia Gravis Composite; MGFA=Myasthenia Gravis Foundation of America; MuSK=muscle-specific tyrosine kinase; NSISTs=non-steroidal immunosuppressive therapies; QMG=Quantitative Myasthenia Gravis; R=randomization; SQ=subcutaneous; TEAEs=treatment-emergent adverse events.
References:
- RYSTIGGO [Prescribing Information]. Smyrna, GA: UCB, Inc.
- Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383-394. doi:10.1016/S1474-4422(23)00077-7
- Data on file. UCB Inc., Smyrna, GA.
- Bril V, Druzdz A, Grosskreutz J, et al. Rozanolixizumab in generalised myasthenia gravis: pooled analysis of the phase 3 MycarinG study and two open-label extensions. J Neuromuscul Dis. 2025;12(2):218-230. doi:10.1177/22143602241205511
- Habib AA, Sacconi S, Antonini G, et al. Efficacy and safety of rozanolizizumab in patients with muscle-specific tyrosine kinase autoantibody-positive generalised myasthenia gravis: a subgroup analysis of the randomised, double-blind, placebo-controlled, adaptive phase III MycarinG study. Ther Adv Neurol Disord. 2024;17:17562864241273036. doi:10.1177/17562864241273036
- Habib AA, Antozzi C, Drużdż A, et al. Efficacy and safety of rozanolixizumab treatment cycles in patients with generalised myasthnia gravis: final pooled analysis of phase 3 studies. Poster presented at: MGFA international 2025; May 13-15, 2025; The Hague, Netherlands. Poster 71.
- Vu T, Antozzi C, Drużdż A, et al. Responder and minimal symptom expression rates with rozanolixizumab in generalised myasthenia gravis: final pooled analysis of phase 3 studies. Poster presented at: MGFA International 2025; May 13-15, 2025; The Hague, Netherlands. Poster 206.
- A study to evaluate rozanolixizumab in study participants with generalized myasthenia gravis. ClinicalTrials.gov identifier: NCT04650854. Updated February 26, 2024. Accessed June 6, 2024. https://clinicaltrials.gov/study/NCT04650854
- A study to investigate the long-term safety, tolerability, and efficacy of rozanolixizumab in adult patients with generalized myasthenia gravis. ClinicalTrials.gov identifier: NCT04124965. Updated September 5, 2023. Accessed June 6, 2024. https://clinicaltrials.gov/study/NCT04124965